
Photobiomodulation (PBM) reduces severity and duration of oral mucositis, and can be used for prevention.
The effect of Photobiomodulation (PBM Therapy) previously known as Low Level Laser Therapy (LLLT) on oral mucositis (by laser or LED) has been reported in 47 randomised controlled clinical trials. These includes patients undergoing chemotherapy, radiotherapy or haematopoietic stem cell transplantation (HSCT) in both pediatric and and adult populations. Most of the studies are therapeutic (treating symptoms) but some clinical trials have shown that treatment concurrent with cancer therapies can be preventative.
PHOTOBIOMODULATION IS HELPFUL FOR:
- Oral mucositis
- Hyposalivation and xerostomia
- Lymphoedema
OTHER EMERGING APPLICATIONS
- Radiation dermatitis
- BRONJ
- Dysphagia
- Trismus
- Fibrosis
- Pain control
CLINICAL TRIAL CONCLUSIONS
Are you a patient? Pass these documents on to your Oncologist
- Download our 'PBM oral mucositis research (PDF)'
- Download our 'PBM radiation dermatitis research (PDF)'
- Download our 'PBM xerostomia, hyposalivation or saliva research (PDF)'
What is PBM ?
Photobiomodulation (PBM) therapy, (previously known as Low Level Laser Therapy or LLLT) is the application of light to tissues to reduce inflammation and improve healing. It works by increasing cellular energy (ATP) and reducing free radicals (oxidative stress). There are over 700 randomised controlled clinical trials published on PBM for a wide range of painful and non healing applications including 47 controlled clinical trials on oral mucositis.
How PBM works
One of the main effects of PBM is on mitochondrial function: There are hundreds of different cell types in the body, each performing different functions, they all contain lots of mitochondria, (cellular power plants) which generate most of the cell's supply of energy (ATP). Mitochondria are also involved in a range of other cellular processes, including signalling, stem cell differentiation, inflammation, cell survival, apoptosis and are consequently implicated in many diseases.
Mitochondria in stressed or ischaemic tissues produce too much mitochondrial nitric oxide (mtNO) which binds to cytochrome c oxidase, competitively displacing oxygen and consequently reducing ATP, but this inhibition of mitochondrial respiration significantly increases production of ROS (free radicals). These excess ROS trigger the process of inflammation, cell death and subsequently oral mucositis.
Light of the correct wavelength (generated by low intensity lasers and LED's), when applied to stressed tissues, is absorbed by cytochrome c oxidase. The light displaces the mtNO thereby reducing oxidative stress and increasing ATP production; this reduces inflammation and increases cell metabolism.
The subsequent cascade of downstream metabolic effects have been shown to include changes in gene transcription (NF-kB and AP-1), activation of latent TGF-β1, increased exchange of Ca2+, secretion of growth factors, activation of enzymes & many secondary messengers.
Within hours (and sometimes minutes) following PBM, increases in cellular activity have been shown in vitro and in vivo in neutrophils, macrophages, fibroblasts, mast cells, endothelial cells and keratinocytes. Reduced inflammatory markers including prostaglandin E2, interleukin 1ß and TNFα have also been seen in many studies.
Clinical trials show a significant improvement in healing of wounds and reduction of pain. A recent systematic review on PBM for OM found that it reduces pain, severity and duration of chemotherapy and radiotherapy induced oral mucositis.
The Multinational Association of Supportive Care in Cancer (MASCC) now recommend PBM for the prevention of oral mucositis in adult patients receiving hematopoietic stem cell transplantation conditioned with high-dose chemotherapy. A new suggestion was made for low-level laser for the prevention of oral mucositis in patients undergoing radiotherapy, without concomitant chemotherapy, for head and neck cancer.
Source: www.mascc.org/mucositis-guidelines
Prices from US$16,000 to US$40,000 (£12,495–£31,995 + VAT)

| CLICK HERE to contact sales | Training course information |

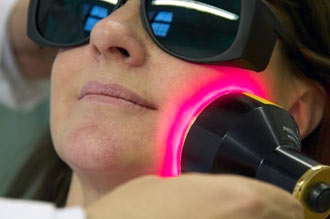
Annette Quinn - Oral Mucositis Academy of Laser Dentistry 2016 View it on YouTube
Product information and purchase enquiry
Sorry for the long form.
We are a global company and our computer needs to know who to direct your enquiry to.
* Mandatory field - this automatically directs your enquiry to the correct department
THOR Photobiomodulation Newsletter
See previous edition. Please add me to the monthly THOR training, research and conference Newsletter.
 Featured Testimonials
Featured Testimonials




"Our results have indicated that the use of low power laser in HSCT patients is a powerful instrument in the treatment of overt OM and is now a standard procedure in this group of patients in our hospital."
"The ease of use of PBM, high patient acceptance, and the positive results achieved, make this therapy feasible for the prevention and treatment of OM in young patients."
"There is consistent evidence from small high-quality studies that red and infrared PBM can partly prevent development of cancer therapy-induced OM. PBM also significantly reduced pain, severity and duration of symptoms in patients with cancer therapy-induced OM."
"Our results indicate that the use of upfront low power laser in patients who have undergone HSCT is a powerful instrument in reducing the incidence of OM and is now standard in our center."
"Laser therapy was effective in preventing and treating oral effects induced by radiotherapy and chemotherapy, thus improving the patient's quality of life."