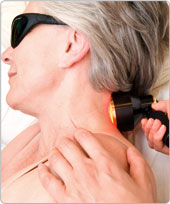
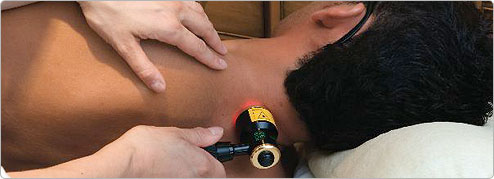
Photobiomodulation relief for neck pain
Systematic review with meta-analysis published by The Lancet
A clinical trial published in the journal PAIN and
acknowledged by the World Health Organisation
NOTE: Treatment videos below and an audio interview to the right.
Results from the recent Lancet review of Low level laser Therapy for chronic neck pain.
The Lancet report declares that neck pain is approaching epidemic proportions with 10 - 24% of the population affected.
Musculoskeletal diseases, which include back pain, arthritis, bodily injuries, and osteoporosis, are reported
by persons in the U.S. more than any other health condition. In 2004, the estimated total cost of treatment and lost wages associated with musculoskeletal diseases was $849 billion, equal to 7.7 percent of the gross domestic product (GDP):
Spine: Low Back and Neck Pain
The Burden of Musculoskeletal Diseases
The paper also reports that pharmacological therapies for neck pain are widely used but have "not shown any conclusive evidence of benefit" The BMJ agrees "There isn't any specific research that shows drugs help neck pain". There is now more evidence for the use of laser for neck pain than any other medical procedure. The Lancet report says "(Relief using) Laser for neck pain lasted for up to 22 weeks. Trials of PBM for knee osteoarthritis tendinopathies and low back pain reported similar results". "This contrasts with drug therapies where the effect ends rapidly when treatment is discontinued."
BRIEF SUMMARY OF LASER STUDIES REVIEWED FOR THE LANCET:
- 16 RCT's of acceptable methodological quality
- Mean duration of symptoms 7.5 years +/-36.9 months
- Mean baseline of pain intensity 56.9mm +/-7.5mm
RESULTS:
- Reduce pain intensity
- Reduced disability
- Reduces recurrence of acute neck pain
- Mean Pain intensity reduction over placebo 20mm (95%CI: 17.1 to 29.8 @ 10-22 weeks)
COMMENT:
This establishes PBM as an evidence based treatment for neck pain. It is at least equivalent to and probably better than other accepted medical treatments for neck pain.
WHAT IS PBM AND HOW DOES IT WORK ON NECK PAIN:
A gentle beam of laser light can stimulate cells to repair tissues, reduce inflammation and inhibit pain fibres to reduce pain. The application of specific wavelengths of red or near infrared laser or LED light on acute injuries and degenerative conditions relieves pain and improves healing. There are two main mechanisms:
- At low doses it acts on mitochondrial proteins to reduce oxidative stress and increase production of ATP.
- At high doses it inhibits fast axonal flow in C and A delta fibres causing a temporary neural blockade, reducing central sensitisation.
HOW LONG IS A TREATMENT SESSION:
Treatment sessions: after initial consultation, actual therapy time takes typically 15 to 20 minutes.
HOW SOON DOES IT START TO WORK:
Patients typically experience improvement (reduction in pain / greater range of movement) immediately after the first treatment.
HOW MANY TREATMENTS DOES IT TAKE:
It can take as much as a dozen sessions but sometimes as few as three.
HOW OFTEN SHOULD PATIENTS COME FOR TREATMENT:
Twice a week for the first two weeks then once a week for a further two weeks.
HOW LONG DO THE BENEFITS LAST:
In most cases, once an acute episode is over, patients are unlikely to need further treatment. For a few people, the benefit may only be temporary - they may need long term maintenance every 6 - 8 weeks.
ADVANTAGES OVER DRUG THERAPIES
The evidence for PBM is much stronger than for drug therapies. There are no analgesics or anti-inflammatories licensed for the treatment of neck pain. PBM also has the advantage of having no side effects, unlike many drug therapies.
PBM NECK PAIN TREATMENT VIDEO
TV INTERVIEW WITH DR ROBERTA CHOW
EQUIPMENT AND TRAINING:
To contact your local dealer for THOR laser systems CLICK HERE.
For training course information CLICK HERE

THOR:
With over 5,000 customers using THOR equipment in 70 countries including Harvard Medical School, NASA researchers, US Navy, RAF, British Army, Royal Navy, NHS, BUPA, premier division football teams, British Lions Rugby, THOR can rightly claim to be the number one supplier of PBM technology and training.
Published in the worlds top scientific medical journals

Over 700 randomised clinical trials and 3,000 laboratory trials.
Dr Roberta Chow Interview
Listen hereOr download sound file
Audio length: 24:35
Dr Chow has given this interview freely to advance the understanding of low level laser therapy in neck pain. This in no way implies a specific endorsement of THOR Photomedicine.
Product information and purchase enquiry
Sorry for the long form.
We are a global company and our computer needs to know who to direct your enquiry to.
* Mandatory field - this automatically directs your enquiry to the correct department
 Featured Testimonials
Featured Testimonials