Photobiomodulation (PBM) for Oncology
What is PBM?
Photobiomodulation (PBM) uses monochromatic light in the red and near-infrared spectrum to improve the speed and quality of tissue repair, reduce inflammation, and relieve pain.
What is PBM for?
NICE recommends PBM to prevent and treat oral mucositis, reducing the incidence of Grade 3 and 4 Oral Mucositis by 70%. THOR's equipment can also be used to treat a number of late effects of cancer treatment.
THOR's equipment improves the speed and quality of repair for dermal and subdermal tissue damaged by treatment. It can reduce the swelling and inflammation that can occur as a result of cancer treatment.
Simple. Quick. Effective.
Treating with the THOR equipment takes minutes. It is so simple that patients can treat themselves.
Acquisition and Implementation
We know that institutional purchases can be bureaucratic and slow. THOR can help. We can also assist you in getting the purchase funded.
THOR's products are proven cost-effective, reducing the use of analgesia, feeding tubes, and unplanned hospital admissions.
For late effects, there are down-the-line savings (related to radiation fibrosis, radiation dermatitis, lymphedema, swallowing, dry mouth and speech).

| CLICK HERE to contact sales | Training course information |
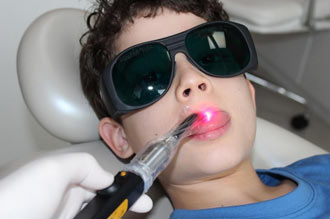

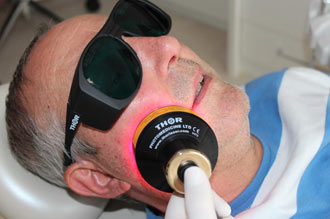
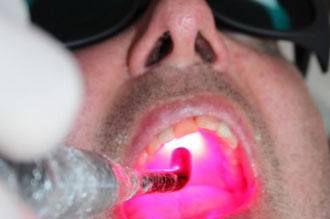
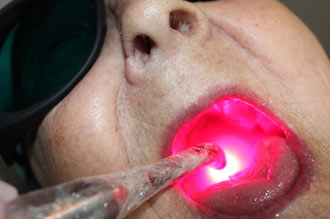
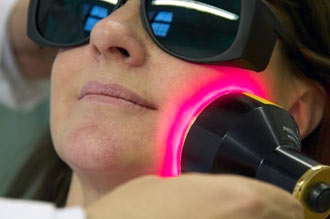
Annette Quinn - Oral Mucositis Academy of Laser Dentistry 2016 View it on YouTube
Product information and purchase enquiry
Sorry for the long form.
We are a global company and our computer needs to know who to direct your enquiry to.
* Mandatory field - this automatically directs your enquiry to the correct department
THOR Photobiomodulation Newsletter
See previous edition. Please add me to the monthly THOR training, research and conference Newsletter.
 Featured Testimonials
Featured Testimonials