A Discussion about Dosage
By James Carroll
There has been a lot of discussion about the best method to quantify dosage.
However, it is my opinion that:
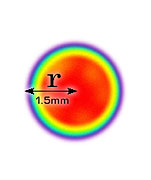
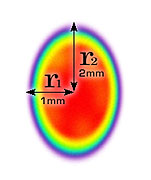
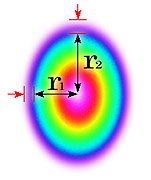
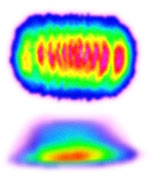
- There is no agreed method of defining beam area
- Dosage expressed as J/cm² is inadequate
No agreed method
|
So beam area is hard to define and there is no agreement in our industry for defining it.
[I propose 1/e² - will explain this soon].
Dosage expressed as J/cm² is inadequate
"Dosage" is usually calculated as Power / Beam Area x Time = J/cm². However, to consider that dosage should equal J/cm² is, in my opinion, inadequate.
Let me explain:
Assume there is a well-defined beam area and thus a quantifiable dosage.
- A 500mW laser with a beam area of 0.25Cm² used for 20 seconds delivers 40 J/cm²
- A 200mW laser with a beam area of 0.1Cm² used for 20 seconds delivers 40 J/cm²
- A 30mW laser with a beam area of 0.015Cm² used for 20 seconds also 40 J/cm²
Each of these probes apparently apply the same "dosage". However, the total energy delivered is clearly different [10 Joules, 4 Joules and 0.6 Joules respectively].
While dosage appears consistent using J/cm², I suggest that the clinical results would be quite diverse. So I say that J/cm² is an inadequate method of expressing dosage.
How to Report Low-Level Laser Therapy (LLLT) / Photomedicine Dose and Beam Parameters in Clinical and Laboratory Studies
Jenkins PA, Carroll JD
BACKGROUND: Dose and beam parameters are critical for successful laser, LED, and other light therapy treatments, however, in our experience, researchers frequently make critical errors and omissions when submitting papers for publication. Journals frequently publish studies with missing data, mathematical errors, and no reported verification of beam parameters. This makes reproducibility impossible, and further confounds an already complex subject.
OBJECTIVE: This article is intended to be a reference document for non-physicist researchers conducting low-level laser therapy (LLLT) laboratory studies and clinical trials to help them design and report the beam and dose aspects of their trials.
RECOMMENDATIONS: It provides a checklist to help LLLT researchers understand and report all the necessary parameters for a repeatable scientific study. It includes the eight most important beam parameters to report, which are: wavelength, power, irradiation time, beam area at the skin or culture surface (this is not necessarily the same as the aperture size), pulse parameters, anatomical location, number of treatments, and interval between treatments. The three commonly used dose parameters are time, energy, and energy density. In addition, more thorough reporting would include coherence, application technique (contact, projection, scanning, pressure), beam profile, and spectral width, as these may also be considered important. Beam power often decreases as the device warms up and as the device ages; therefore, this should be checked routinely during an experiment/trial. Measurements of beam area and beam power require special instruments and trained technicians to operate them. Power measurements should be taken before, after, and at frequent intervals during research trials.
CONCLUSION: Reviewers should insist that the minimum eight most important beam parameters are included and authors should take care to measure and record these accurately before during and after an experiment or clinical trial.
Photomed Laser Surg 2011 Nov 22
 Featured Testimonials
Featured Testimonials