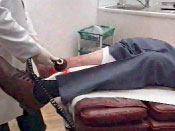
THOR Photobiomodulation for Wound Healing
"It is simple to use, very cost-effective in terms of finance and time,
and comes in its own case for easy transport between patients. It is
used in conjunction with the patient's dressing regime"
See the full article by Tissue Viability Nurse, Lydia Jack
See the Treatment Video »
"Nurses who treat wounds should have access to PBM equipment. It would be a cost effective method of improving the quality of life of many patients"
See the full article by biologist Dr Mary Dyson
 "Our preliminary experience has been overwhelmingly positive. The THOR DD
II device is very simple to use and calibrate and is quite predictable in its
function and effect. Wounds are healing more rapidly than, we have noted, in
work with other devices..., although the wounds are indeed healing with PBM
applied using the appropriate parameters/dosimetry. It is our belief that
these devices are an important adjunct. My personal bias would be to apply
this technology to patients early; that is, to treat them at the first sign
of a wound or injury. We are continuing our clinical and laboratory
investigations aimed at elucidating the mechanisms and utility of
photobiomodulation in humans."
"Our preliminary experience has been overwhelmingly positive. The THOR DD
II device is very simple to use and calibrate and is quite predictable in its
function and effect. Wounds are healing more rapidly than, we have noted, in
work with other devices..., although the wounds are indeed healing with PBM
applied using the appropriate parameters/dosimetry. It is our belief that
these devices are an important adjunct. My personal bias would be to apply
this technology to patients early; that is, to treat them at the first sign
of a wound or injury. We are continuing our clinical and laboratory
investigations aimed at elucidating the mechanisms and utility of
photobiomodulation in humans."
 Featured Testimonials
Featured Testimonials