How does PBM / Photobiomodulation Therapy work?
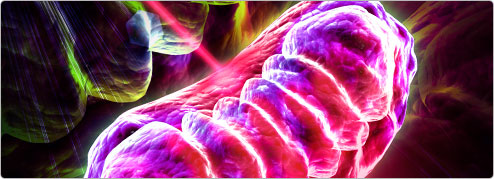 Photobiomodulation (PBM Therapy) previously known as Low Level Laser Therapy (LLLT) has a photochemical effect (like photosynthesis in plants). One of the main mechanisms of action occurs in the mitochondria (the cellular power plant inside every cell). The effect depend on the application of the correct wavelength and density of light, delivered to the target tissues for an appropriate period of time (typically between 30 - 60 seconds). Pulses can improve tissue repair and anti-inflammatory effect, analgesia is best achieved with a continuous beam.
Photobiomodulation (PBM Therapy) previously known as Low Level Laser Therapy (LLLT) has a photochemical effect (like photosynthesis in plants). One of the main mechanisms of action occurs in the mitochondria (the cellular power plant inside every cell). The effect depend on the application of the correct wavelength and density of light, delivered to the target tissues for an appropriate period of time (typically between 30 - 60 seconds). Pulses can improve tissue repair and anti-inflammatory effect, analgesia is best achieved with a continuous beam.
TISSUE REPAIR AND ANTI-INFLAMMATORY EFFECTS
The primary effect occurs when light is absorbed in cytochrome c oxidase a protein within the mitochondria.
When cells get stressed (perhaps due to disease, injury or ageing) the mitochondria produces nitric oxide (NO). This competitively displaces oxygen from cytochrome c oxidase consequently reducing ATP (an essential intracellular cellular energy and extracellular signalling molecule) and causing an over production reactive oxygen species (ROS) and leading to oxidative stress. Oxidative stress is well known to lead to inflammation an cell death via the gene transcription factor NF-Kb.
PBM of the correct wavelength and density, dissociates NO allowing oxygen back in, so ATP is restored and oxidative stress reduced. Once normal mitochondrial function is restored by PBM then cell metabolism is improves, and the patient gets better more quickly.
What is PBM?
How does PBM Work?
What are the benefits of PBM?
What are the contra-indications of PBM?
3B vs Class IV Lasers
Calculating PBM Dosage
Request more Information
Laser Training Courses
Product information and purchase enquiry
Sorry for the long form.
We are a global company and our computer needs to know who to direct your enquiry to.
* Mandatory field - this automatically directs your enquiry to the correct department
 Featured Testimonials
Featured Testimonials