3B vs Class IV Lasers
Laser overdose - How much is enough? How much is too much ?
Lies, damn lies and no statistics (they call it marketing)
|
How much laser is enough? and how much is too much ?
In a recent systematic review and meta analysis of laser therapy on tendinopathies
(Tumilty et al), 11 out of 20 studies failed to produce a positive result. The reason identified
for the ineffective studies were that the laser beams were too strong (the irradiance was
too high for this particular pathology). Most clinicians are rightly confused about which
laser to choose and which dose or technique will work.
Read full post
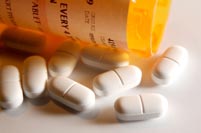
LLLT companies may be no better than Big Pharma
It is a popular sport in the LLLT industry to sneer at Big Pharma for their side effects and
marketing practices, but the LLLT industry is far from criticism itself. Whilst LLLT side
effects are hard to find, marketing overstatement and misdirection are common place.
In July's literature watch is a paper titled "The Effectiveness of Therapeutic Class IV
(10 W) Laser Treatment for Epicondylitis". This small study showed that 10 Watt Class IV
laser (mixed 8W 970nm, 2W 810) was successful in reducing pain and improving function
in an RCT with 15 patients, and that there was good statistical significance at 6 months
following a course of 6 treatments.
Read full post
What is PBM?
How does PBM Work?
What are the benefits of PBM?
What are the contra-indications of PBM?
3B vs Class IV Lasers
Calculating PBM Dosage
Request more Information
Laser Training Courses
Product information and purchase enquiry
Sorry for the long form.
We are a global company and our computer needs to know who to direct your enquiry to.
* Mandatory field - this automatically directs your enquiry to the correct department
 Featured Testimonials
Featured Testimonials