"Dosage" is a difficult subject. Why ?
4 things you should know about PBM laser beam measurement and dosage
James Carroll - THOR lasers
1. Wavelength
1.1 Diode laser wavelength is rarely as claimed on product labels and the wavelength changes with temperature.
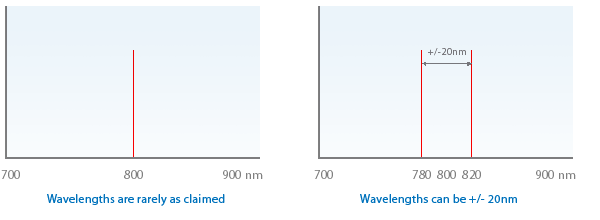
1.2 Unless actually specified wavelengths can be +/- 20nm (we routinely specify +/- 5nm)
1.3 Wavelength shifts with temperature - typically 0.3nm per 1 ºC (the diode temperature may rise in a treatment session 5 - 20º)
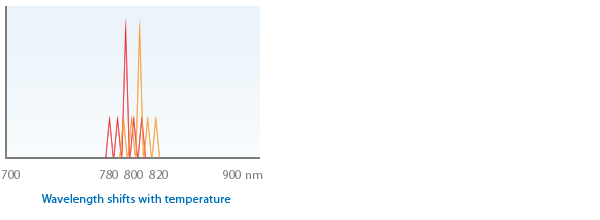
If you believe that wavelength is critical to your research at operating temperature then try to stabilize temperature
2. Power
2.1 Diode laser output power is rarely as claimed on the product labels and it also changes with temperature.
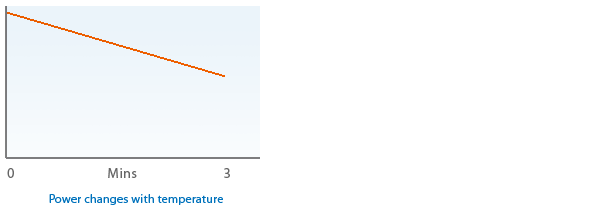
2.2 If you believe that dosage is critical to your research have your laser power tested before, during and after procedures at operating temperature then try to stabilize temperature
3.1 Diode laser beams are hard to measure. There has never been an agreed method for the last 40 years and comparing research between different centres is impossible. The most common formula is J/cm2.
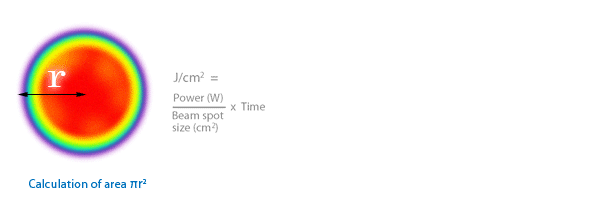
3.2 However, they are almost never round, collimated, or homogeneous.
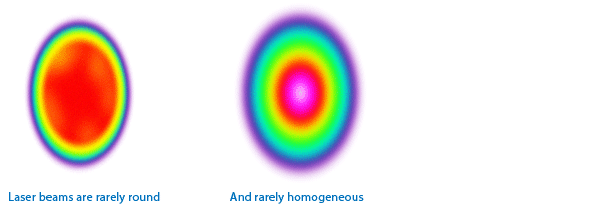 At best they can be described as being elliptical and having a gaussian distribution.
At best they can be described as being elliptical and having a gaussian distribution.
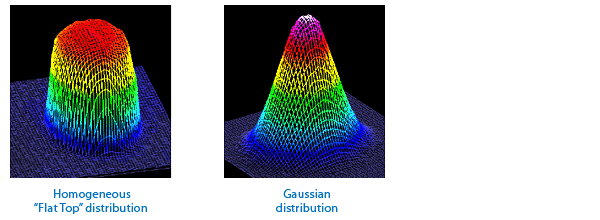
3.3 How do we measure and calculate the area of elliptical, gaussian distribution beam?

3.4 But shouldn't we take into account the small amount of light on the outer edges?

What happens if we include that small amount of light in our area calculation?

In theory, our beam measurment drops from 48 J/cm2 to 25 J/cm2 - approxiamately half of our original calculation when we factor in the outer low-light areas.
3.5 To make matters worse, laser beams are not always Elliptical or Gaussian.
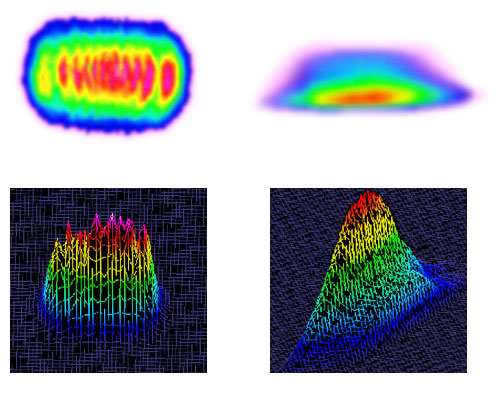
3.6 Use the appropriate instrument to measure beam area.
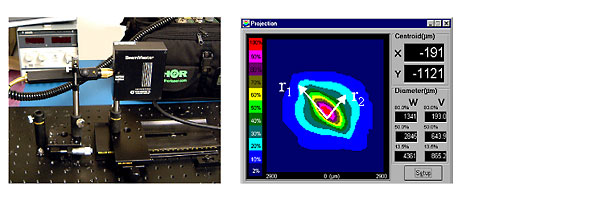
If you believe that intensity (irradiance) and Energy Density “dosage” (J/cm2) is critical to your research
- Have your laser beam area measured with professional beam measurement equipment
- Use 1/e2 point
- Publish the measurement method used
- Be very skeptical of J/cm2 or intensity quoted by previous authors
4. Dosage
4.1 Reporting Joules only is an inadequate expression of dosage. If we increase the power ten times and reduce the time to one tenth we have the same Joules but will we get the same result?
| 500mW x 10sec = 5J | |
| 10mW x 500sec = 5J |
The calculations above are the same “dosage” but they would produce different results in a clinical situation.
A 1mW laser, with a 0.001cm2 beam area used for 4 second = 4J/cm2
A 500mW laser with 1cm2 beam area used for 8 seconds = 4J/cm2
| 500mW 1cm2 |
x 8sec = 4J/cm2 [4 Joules total] |
| 1mW 0.001cm2 |
x 4sec = 4J/cm2 [0.004 Joules total] |
4.2 Conclusions
- J/cm2 is a grossly inadequate method of reporting dosage.
- They are both the same “dosage” but will you get the same result?
- If we believe that these parameters are important how should we measure and report them?
How to Report Low-Level Laser Therapy (LLLT) / Photomedicine Dose and Beam Parameters in Clinical and Laboratory Studies
Jenkins PA, Carroll JD
BACKGROUND: Dose and beam parameters are critical for successful laser, LED, and other light therapy treatments, however, in our experience, researchers frequently make critical errors and omissions when submitting papers for publication. Journals frequently publish studies with missing data, mathematical errors, and no reported verification of beam parameters. This makes reproducibility impossible, and further confounds an already complex subject.
OBJECTIVE: This article is intended to be a reference document for non-physicist researchers conducting low-level laser therapy (LLLT) laboratory studies and clinical trials to help them design and report the beam and dose aspects of their trials.
RECOMMENDATIONS: It provides a checklist to help LLLT researchers understand and report all the necessary parameters for a repeatable scientific study. It includes the eight most important beam parameters to report, which are: wavelength, power, irradiation time, beam area at the skin or culture surface (this is not necessarily the same as the aperture size), pulse parameters, anatomical location, number of treatments, and interval between treatments. The three commonly used dose parameters are time, energy, and energy density. In addition, more thorough reporting would include coherence, application technique (contact, projection, scanning, pressure), beam profile, and spectral width, as these may also be considered important. Beam power often decreases as the device warms up and as the device ages; therefore, this should be checked routinely during an experiment/trial. Measurements of beam area and beam power require special instruments and trained technicians to operate them. Power measurements should be taken before, after, and at frequent intervals during research trials.
CONCLUSION: Reviewers should insist that the minimum eight most important beam parameters are included and authors should take care to measure and record these accurately before during and after an experiment or clinical trial.
 Featured Testimonials
Featured Testimonials